Powering the next generation in bioelectric medicine with nPulse™ technology.
Welcome
Pulse Biosciences pioneers in the research, design and production of our patented nPulse™ technology for use in high impact areas of healthcare. We are currently focused on applying our proprietary nPulse technology for the ablation of soft tissue and related applications, such as the ablation of benign thyroid tumors and nodules as well as ablations to restore normal cardiac sinus rhythm such as atrial fibrillation.
The nPulse™ platform using nsPFA™ energy is a novel technology that has the potential to not only help patients requiring soft tissue ablation such as benign thyroid nodule ablation and with atrial fibrillation but also patients across many different therapy areas.
Forward-Looking Statements
Our website and newsfeed contain forward-looking statements such as statements relating to the anticipated safety and effectiveness of our nPulse™ technology, statements concerning the Company’s expected product development efforts, statements about possible government clearances and approvals, and statements concerning market opportunities, customer adoption and future use of the nPulse™ System to address a range of conditions such as atrial fibrillation. These statements are not historical facts but rather are based on our current expectations, estimates, and projections. You should not place undue reliance on forward-looking statements because they involve risks, uncertainties, and assumptions that are difficult or impossible to predict. Actual results may differ materially from our forward-looking statements for a number of reasons, including those described in our filings with the SEC, which you should read before making an investment in our Company.
Latest News
Jan 9, 2026

Pulse Biosciences, Inc. Appoints Maria Sainz to its Board of Directors
Pulse Biosciences, Inc. today announced the appointment of Maria Sainz to its Board of Directors effective as of January 9, 2026. Maria is a distinguished leader in the medical technology sector, bringing over 30 years of experience and a remarkable track record of scaling both public and private companies. Currently serving as President and CEO of Hyperfine, Inc., Maria’s deep expertise in medical device markets and commercial strategy arrives at a pivotal moment for Pulse Biosciences.
May 21, 2025
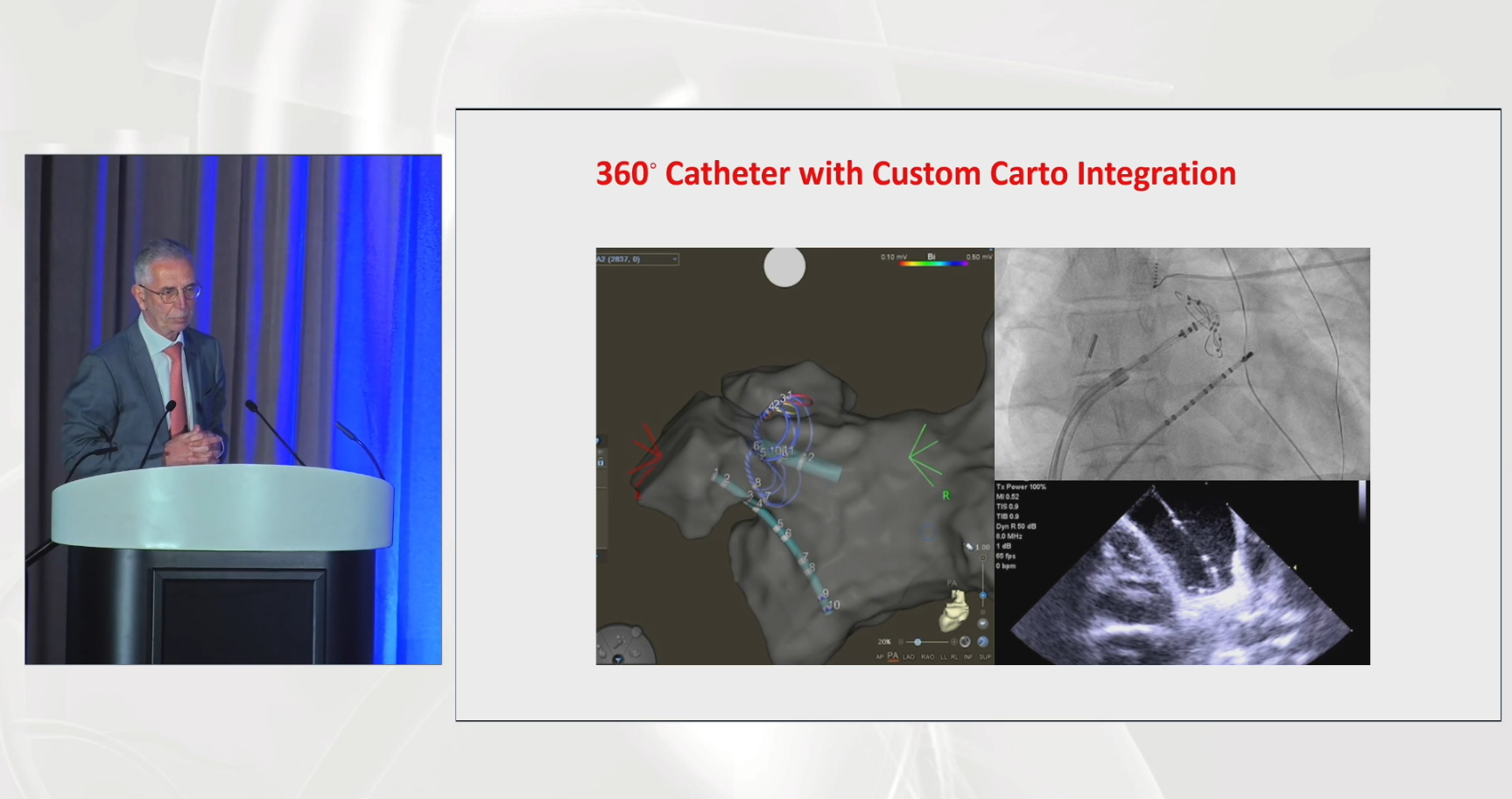
Pulse Biosciences Presents Live Case at HRS 2025
Live case at HRS 2025 showcasing the nsPFA™ 360˚ Catheter’s ability to rapidly isolate the pulmonary veins, integrated with the CARTO™ 3 Mapping System from Johnson & Johnson,performed by Dr. Jan Petru and Dr. Moritoshi Funasako and moderated by Dr. Vivek Reddy, Dr. Petr Neuzil and Dr. Gregory Michaud who provided additional commentary on use of the nsPFA 360˚ Catheter.
CAUTION—Investigational device. Limited by Federal (or United States) law to investigational use.
February 5, 2025

Pulse Biosciences Presents Live Case at AF Symposium 2025
Live case highlighting the nsPFA 360 Catheter’s ability to rapidly isolate the pulmonary veins with a fast, efficient workflow performed by Prof. Petr Neuzil and Drs Moritoshi Funasako and Jan Petru and moderated by Dr. Vivek Reddy providing additional commentary on the utility of the nsPFA 360 Catheter from his experience.
Caution: Exclusively for Clinical Investigations.
October 30, 2024

Pulse Biosciences Announces Appointment of David Kenigsberg, M.D. as Chief Medical Officer of Electrophysiology
Dr. David Kenigsberg joins Pulse Biosciences as Chief Medical Officer of Electrophysiology. Dr. Kenigsberg is a highly respected cardiac electrophysiologist with a profound commitment to advancing treatments for atrial fibrillation.
February 14, 2024
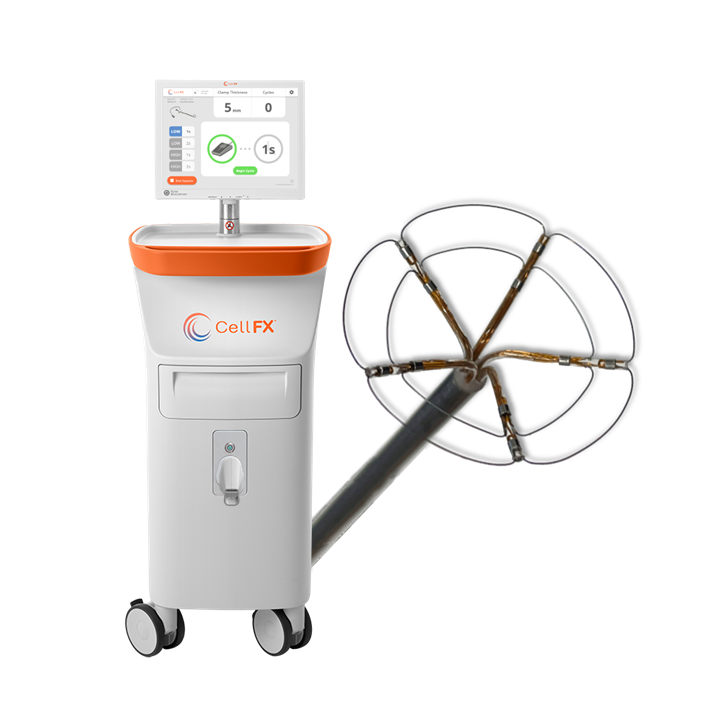
Pulse Biosciences announces promising results from First-in-Human CellFX® nsPFA™ 360 Cardiac Catheter Study
Pulse Biosciences announced today promising results from the 60-day follow-up evaluations of the initial patients treated in the CellFX® nsPFA™ 360 Cardiac Catheter First-in-Human Feasibility Study. This landmark study, utilizing our novel and proprietary Nanosecond Pulsed Field Ablation (nsPFA) technology for atrial fibrillation treatment, has demonstrated favorable durability of pulmonary vein isolation, a critical success factor in AF management. Led by Dr. Vivek Reddy of the Mount Sinai Fuster Heart Hospital, NY, these findings mark a significant step forward in our commitment to advancing cardiac care. With 14 patients already treated and plans to expand, Pulse Biosciences is at the forefront of developing innovative solutions for electrophysiology and cardiac health.
February 3, 2024

Prof. Stefano Spiezia Presents on nPulse™ Technology at NASIT 2024
Prof. Stefano Spiezia presented groundbreaking data from the first-in-human clinical study conducted in Naples at the recent NASIT Annual Meeting 2024. This pioneering research focuses on the application of the nPulse™ Vybrance™ Percutaneous Electrode in the treatment of benign thyroid nodules.
Prof. Spiezia’s presentation shed light on the significant findings and potential implications of this innovative therapy, offering a new horizon in the nonthermal, minimally invasive treatment of thyroid nodules. 30 patients have been treated in the study, which is to determine the efficacy and safety of the nPulse technology for benign thyroid nodules, positioning it as a promising alternative to conventional treatments with the potential to revolutionize patient care for benign thyroid nodules. The nPulse™ Vybrance™ Percutaneous Electrode is not cleared by FDA and is for investigational use only.
February 2, 2024
Dr. Vivek Reddy Presents First-In-Human Studies for CellFX® nsPFA™ 360 Cardiac Catheter
Dr. Vivek Reddy participated at the 29th Annual AF Symposium 2024, where he delivered a compelling presentation on the CellFX® nsPFA™ 360 Cardiac Catheter. Dr. Reddy’s presentation meticulously outlined the cutting-edge differences and potential advantages of the CellFX® nsPFA™technology over conventional microsecond PFA devices, emphasizing its potential to significantly enhance atrial fibrillation therapies.
The Company takes this opportunity to express its appreciation to Dr. Reddy for his expert contribution and to acknowledge the essential role of the clinical team, whose dedication and hard work were instrumental in facilitating this event. The presentation was met with enthusiastic participation from attendees, underscoring the medical community’s interest in innovative solutions for atrial fibrillation treatment.
This event marks a significant milestone in the ongoing efforts to provide advanced therapeutic options to patients worldwide, reinforcing Pulse Biosciences’ position at the forefront of medical innovation and the potential treatment of atrial fibrillation. The CellFX® nsPFA™ 360 Cardiac Catheter is not approved by FDA and is for investigational use only.
January 2, 2024
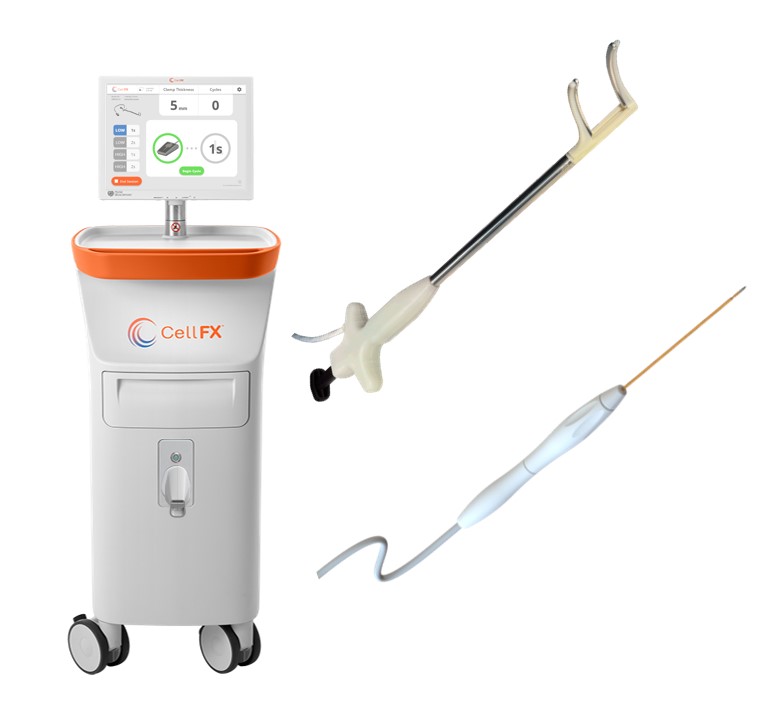
Pulse Biosciences Submits Two Devices for 510(k) Clearance to US FDA
Pulse Biosciences has recently announced two significant 510(k) submissions to the U.S. Food and Drug Administration (FDA), marking important milestones in the company’s expansion of its CellFX Nanosecond Pulsed Field Ablation (nsPFA) technology into new medical fields. The first submission, for the CellFX® nsPFA™ Cardiac Clamp, represents a novel approach to surgical cardiac ablation, designed to produce continuous linear transmural ablations during cardiac surgical procedures. This technology promises to deliver significant safety and performance benefits over existing thermal ablation technologies, potentially reducing procedure times and minimizing the risk of collateral damage to adjacent tissues. The company’s leadership expressed confidence in the clamp’s ability to set new standards in cardiac ablation, highlighting its nonthermal mechanism and the impressive preclinical data supporting its efficacy and safety.
In addition to the cardiac focus, Pulse Biosciences has also filed a 510(k) for its innovative CellFX® nsPFA™ percutaneous electrode, aimed at non-cardiac applications. This needle device leverages the precision of nsPFA energy to achieve complete nonthermal ablation of cellular tissue, sparing noncellular structures. The submission of these two 510(k)s, underscores the company’s commitment to advancing medical treatment options across a broad spectrum of applications. Pulse Biosciences’ leadership expressed enthusiasm for the potential for these technologies to significantly impact patient care and outcomes, signaling a robust growth trajectory for the company’s proprietary nsPFA technology in both cardiac and non-cardiac fields. Neither device has yet been cleared by FDA and are for investigational use only.
Oct 24, 2023

Pulse Biosciences Appoints Dr. Niv Ad as Chief Science Officer, Cardiac Surgery
“After working with the Pulse Biosciences’ team and participating in preclinical studies using the nsPFA clamp for cardiac ablation, it was immediately clear to me that nsPFA has the potential to not only replace all other energy modalities in cardiac ablation, including radiofrequency and cryo, but due to the speed, safety, and ablation performance of the system, it has the potential to significantly expand the number of patients we treat in cardiac surgery,” said Dr. Niv Ad, Chief Science Officer, Cardiac Surgery. “Because of this and how far along the Company is in the development of a commercially viable system, I decided to join this experienced team as it enters this next exciting phase of bringing CellFXTM Technology from the lab to the clinic to make a positive impact on patient lives and healthcare. I look forward to working alongside this impressive team.”
Dr. Ad is a leading authority in surgical treatment of atrial fibrillation, minimally invasive heart surgery and other advanced heart surgery techniques and transcatheter therapies. Dr. Ad has published more than 200 research articles in peer-reviewed publications. He has led the development of national and international clinical practice guidelines for the different cardiovascular societies for ISMICS, AATS and STS, and serves on multiple leadership positions and committees in the leading cardiothoracic societies. He is the past president of The International Society of Minimally Invasive Cardiothoracic and Vascular Surgery (MICS), the leading entity for minimally invasive procedures globally and he is the Editor in Chief of INNOVATIONS: Technology and Techniques in Cardiothoracic and Vascular Surgery.
Oct 21, 2023
Pulse Biosciences Appoints Dr. Niv Ad as Chief Science Officer, Cardiac Surgery
Pulse Biosciences achieved the successful completion of the first five procedures using our proprietary CellFXTM Nanosecond Pulsed Field Ablation (nsPFA) cardiac catheter in a first-in-human feasibility study for atrial fibrillation treatment. This significant accomplishment took place at Na Homolce Hospital in Prague, with the help from Dr. Jacob Koruth, Dr. Iwanari Kawamura, Dr. Moritz Nies, Dr. Kaita Watanabe, Dr. Vivek Reddy, Dr. Moritoshi Funasako, Dr. Petr Neuzil, and the rest of the Na Homolce Team, in particular Stepan Kralovec, who helped guide the process and was instrumental in making this First-In-Human the experience it was.
Dr. Reddy, Director of Cardiac Arrhythmia Services at Mount Sinai Hospital, NY, shared, “We are excited to report that our experience with these first five patients has validated our belief that this may represent the next generation of PFA technology for the treatment of AF. The speed and ease with which we were able to isolate the pulmonary veins with the nsPFA 360 catheter was impressive, and all patients tolerated the procedure well.”
May 23, 2023
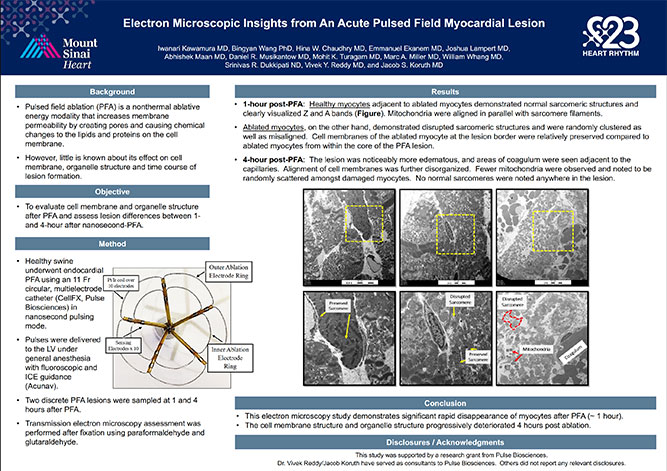
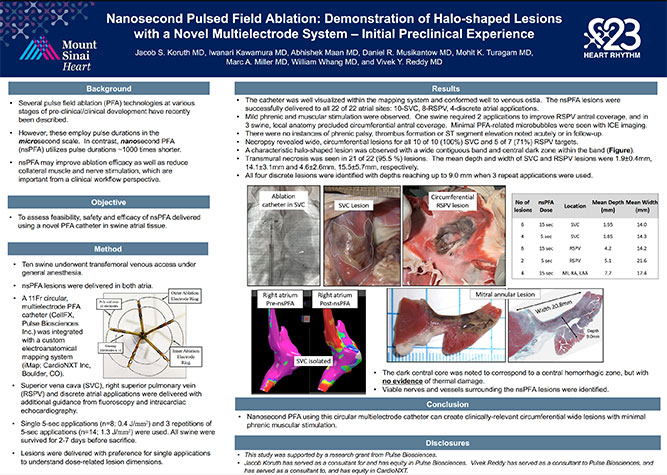
Pulse Biosciences’ proprietary Nanosecond Pulsed Field AblationTM (nsPFATM) Technology was recently featured in multiple presentations at the 2023 Heart Rhythm Society Meeting (HRS 2023). These presentations demonstrated preclinical evidence on the use of nsPFA energy for the treatment of heart rhythm disorders. “This data is a validation of the quality of the preclinical work completed with our physician collaborators and our opportunity to advance the treatment of atrial fibrillation. We are excited to continue on the path towards first in-human clinical use,” said Kevin Danahy, Chief Executive Officer.
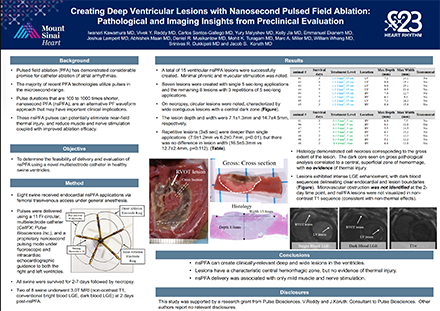
Creating Deep Ventricular Lesions with Nanosecond Pulsed Field Ablation: Pathological and Imaging Insights from Preclinical Evaluation (Iwanari Kawamura MD, et al)
Feb 2, 2023
Pulse Biosciences announces poster presentation at the 2023 AF Symposium describing the novel use of Nanosecond Pulsed Field Ablation (nsPFA) technology in cardiac ablation for the treatment of arrhythmias. View press release.